Understanding Electroplating: How It Works and Its Applications
What Is Electroplating
Electroplating is a fascinating process that uses an electric current to deposit a thin layer of metal onto the surface of another material. This technique is widely utilized in various industries due to its ability to significantly enhance the properties of the base material.
The primary applications of electroplating include:
- Preventing corrosion: By adding a protective metal layer, the underlying material is shielded from corrosive elements.
- Increasing wear resistance: Electroplating can make surfaces harder and more resistant to abrasion, extending the lifespan of components.
- Improving appearance: Aesthetic appeal is often enhanced, with electroplated surfaces boasting a shiny, polished finish that is visually appealing.
- Reducing friction: Certain electroplated surfaces offer lower friction, which is beneficial in mechanical parts and systems.
This process not only improves the durability and functionality of materials but also adds to their aesthetic value, making it indispensable in fields ranging from automotive to jewelry.
How Does Electroplating Work
1. Surface Preparation
The first crucial step in electroplating is cleaning the substrate surface to ensure good adhesion of the metal coating. Any dirt, oil, or oxidation on the surface can hinder the electroplating process, so thorough cleaning is essential.
2. Electrolyte Solution Preparation
Next, the electrolyte solution is prepared. This solution contains the metal ions that will be deposited onto the substrate. The choice of metal ions depends on the desired properties of the electroplated layer, whether it’s for increasing wear resistance or improving appearance.
3. Electroplating
During the electroplating process, the object to be plated is connected to the negative terminal (cathode) of a power supply, and the metal anode (the metal that will be deposited) is connected to the positive terminal. When the electric current is applied, metal ions in the electrolyte solution are reduced and deposited onto the cathode through electrolysis.
4. Post-treatment
The final step involves post-treatment procedures such as rinsing, passivation, and other finishing steps to enhance the properties of the plated layer. These treatments help to ensure the durability and effectiveness of the electroplated coating, providing the desired benefits such as preventing corrosion and reducing friction.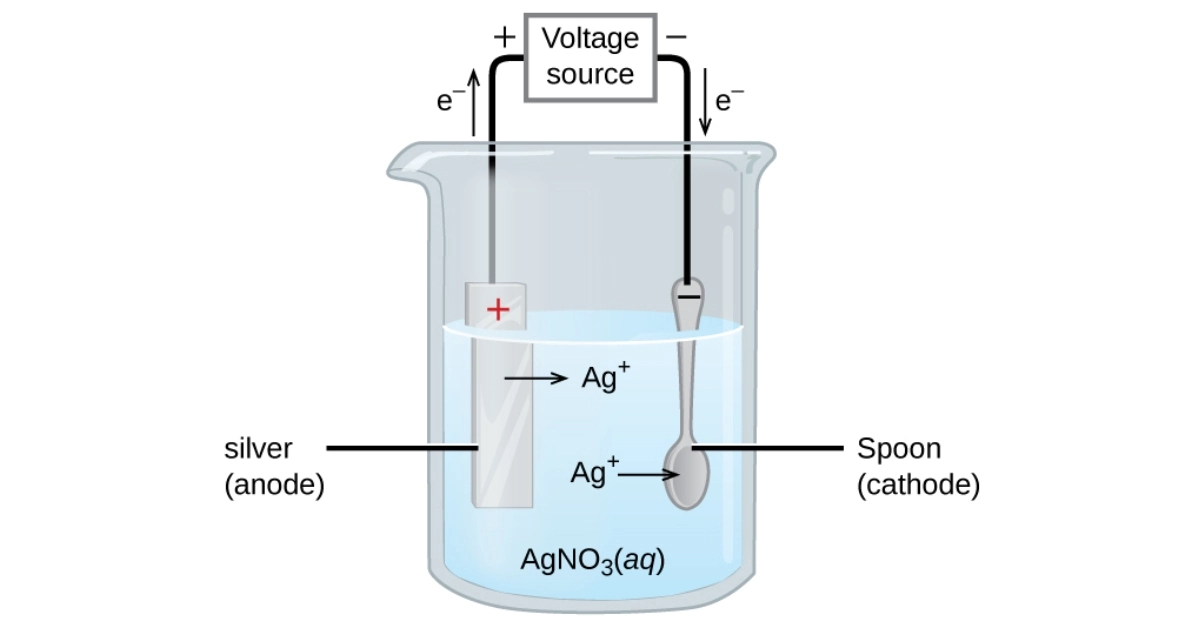
This systematic approach ensures a high-quality and consistent electroplated finish, making it a reliable method for improving both the functionality and aesthetics of various materials.
Common Types of Electroplating and Electroplating Colors
The various types of electroplating are primarily categorized by the metal used in the plating process. Each metal creates a coating with unique characteristics and colors, tailored to specific applications and needs.
- Zinc Plating: Zinc plating is widely used to prevent corrosion of steel and iron. By applying a thin layer of zinc to these metals, the underlying material is protected from rust and environmental damage, extending its lifespan significantly. The color of electroplated zinc can exhibit a bluish or yellowish tint, depending on the post-treatment processes applied.
- Nickel Plating: Nickel plating is renowned for providing excellent corrosion resistance and a bright, attractive finish. This type of electroplating is often used in applications where both appearance and durability are important, making it a popular choice for automotive parts, household fixtures, and more. Electroplated nickel results in a shiny, silvery finish that is both durable and visually appealing.
- Chrome Plating: Chromium electroplating is primarily used for its decorative finishes and hard, wear-resistant surfaces. The reflective, shiny surface of chrome-plated objects not only looks appealing but also offers superior hardness and resistance to abrasion, making it ideal for automotive trim, tools, and other high-wear applications. Hard chrome electroplating, in particular, is used to enhance the durability of industrial components, providing a thick, robust coating that can withstand extreme wear and tear.
- Gold and Silver Plating: Gold and silver plating are prized for their high conductivity and decorative finishes. Electroplated gold typically boasts a rich, warm yellow hue, while electroplated silver provides a bright, white sheen. These precious metals are often electroplated onto electronic components to ensure reliable conductivity and onto jewelry and decorative items to enhance their aesthetic appeal.
- Copper Plating: Copper electroplating produces a distinctive reddish-brown color and is frequently used as an intermediate layer in multi-layer plating processes. It provides excellent conductivity and is often used in printed circuit boards and electrical components.
- Rhodium Plating: Rhodium electroplating is prized for its reflective, tarnish-resistant surface. It is often used to plate jewelry, giving it a brilliant, long-lasting finish.
- Palladium Plating: Palladium electroplating typically produces a white, shiny finish provides excellent corrosion resistance, and is used in electronics, jewelry, and dental applications. Its ability to withstand harsh environments makes it a valuable material for various industries.
The choice of electroplating colors and types depends on the desired aesthetic and functional properties of the finished product. This makes electroplating a highly customizable and versatile process, capable of meeting a wide range of needs and preferences.
Can Electroplating Be Done on Plastic
Yes, electroplating can indeed be done on plastic. This process, often referred to as plastic electroplating, involves several additional steps to prepare the non-conductive plastic surface to accept a metal coating. Since plastic is not conductive, the first step in plastic electroplating is to thoroughly clean and etch the plastic surface to ensure good adhesion. This typically involves a series of chemical treatments that roughen the surface at a microscopic level, creating a texture that the metal can adhere to.
After preparing the surface, a thin layer of conductive material, such as special conductive paint or a thin layer of electroless plating, is applied to the plastic part. This conductive layer allows the electroplating process to proceed as it would on a metal surface. Once the plastic part has this conductive layer, it undergoes the same electroplating process as metal parts. Electroplated plastic parts can have the same corrosion resistance, wear resistance, and conductivity as their metal counterparts, making this a versatile and valuable process in manufacturing.
Does an Electroplated Layer, Such as Gold Electroplate, Tarnish?
Electroplated layers, including gold electroplate, can indeed tarnish over time, depending on various factors. While gold itself is highly resistant to tarnishing, the underlying metals and the quality of the electroplating process play crucial roles in determining the durability of the finish.
Gold electroplating typically involves a thin layer of gold applied over a base metal. If the gold layer is too thin or if the base metal is prone to oxidation, tarnishing can occur as the underlying metal reacts with the environment. High-quality electroplating processes can mitigate this by ensuring a thicker and more uniform gold layer, which provides better protection against tarnishing.
In general, other types of electroplated layers can also tarnish. Metals like silver, copper, and nickel are more susceptible to tarnishing when exposed to air and moisture. The rate at which tarnishing occurs depends on the specific metal and environmental conditions. For instance, nickel plating can develop a dull finish over time, and silver plating can form a black tarnish layer due to sulfur compounds in the air.
Proper maintenance and care can extend the life of any electroplated item. Regular cleaning and avoiding exposure to harsh chemicals can help maintain the luster and prevent tarnish, ensuring the electroplated layer remains attractive and functional for longer.
Difference Between Electroplating And Anodizing
Electroplating and anodizing are both surface treatment processes used to enhance the properties of metal objects, but they differ significantly in their methods and outcomes.
Electroplating is a process that uses an electric current to deposit a thin layer of metal onto the surface of another material. This method is primarily used to improve the appearance, corrosion resistance, and wear resistance of the substrate. Common metals used in electroplating include gold, silver, nickel, and zinc. During the electroplating process, the object to be plated is submerged in an electrolyte solution containing metal ions, and an electric current causes these ions to adhere to the object’s surface, forming a uniform coating.
Anodizing, on the other hand, is an electrochemical process that converts the metal surface into a durable, corrosion-resistant oxide layer. Unlike electroplating, which adds a new layer of metal to the surface, anodizing enhances the existing surface by increasing the thickness of the natural oxide layer. This process is most commonly used with aluminum, but can also be applied to titanium and magnesium. During anodizing, the metal object is submerged in an acid electrolyte bath and subjected to an electric current, which causes the surface to oxidize and form a protective, porous layer that can also be dyed in various colors.
In summary, the primary difference between electroplating and anodizing lies in their objectives and methods: electroplating deposits a new metal layer onto the surface to enhance its properties, while anodizing transforms the surface itself to create a protective oxide layer.
Applications of Electroplating
Electroplating is extensively used across various industries to enhance the performance and longevity of components. In the automotive sector, it improves the corrosion resistance and durability of automotive parts, while in electronics, it ensures excellent conductivity and oxidation prevention for electronic components. The aerospace industry relies on electroplating to protect metal parts from extreme conditions, and in the medical field, it ensures biocompatibility and durability of medical devices. Additionally, electroplating 3D prints enhances the durability, conductivity, and appearance of 3D-printed objects, making them suitable for a wide range of applications, including prototypes and functional parts. In everyday life, electroplating is used to give jewelry, decorative items, and kitchen utensils a polished, durable finish, improving their aesthetic appeal and resistance to wear and corrosion.
HDC’s Electroplating Services
At HDC, we offer a wide range of electroplating options to enhance the custom products we manufacture to meet the diverse needs of our clients. Whether you have custom metal or plastic products, our advanced electroplating services can enhance their durability, appearance, and functionality. We provide tailored solutions to ensure that each product meets the highest standards of quality and performance. From gold and silver plating for decorative items to nickel and chrome plating for industrial components, HDC is equipped to deliver superior finishes that add value and longevity to your custom-made items. Our expertise in electroplating ensures that your products not only look great but also stand up to the rigors of their intended use.
Conclusion
Electroplating is a versatile and essential process used across various industries to enhance the durability, functionality, and appearance of metal and plastic components. From improving corrosion resistance and wear resistance to achieving aesthetically pleasing finishes, electroplating plays a crucial role in the automotive, electronics, aerospace, and medical fields, as well as in everyday items like jewelry and kitchen utensils.
Discover more with our blog posts.
Recent Posts
Discover more about our products
HDC Products
Instant Quote!









